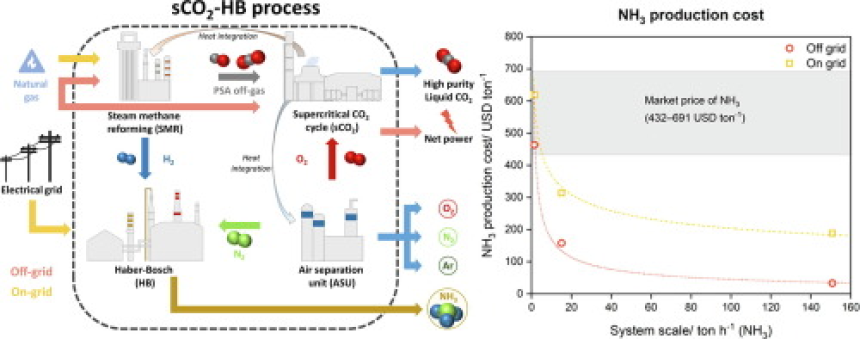
The Haber-Bosch Process
The Haber-Bosch Process: An Essential Part of Modern Agriculture
The Haber-Bosch Process: An Essential Part of Modern Agriculture
The Haber-Bosch Process is a crucial chemical process that plays a significant role in modern agriculture. Named after its developers Fritz Haber and Carl Bosch, this process converts nitrogen gas from the air into ammonia, a compound rich in nitrogen that is used to produce fertilizers and other important chemicals. In this blog, we will explore the Haber-Bosch Process in detail and highlight its importance in boosting global food production.
Understanding the Haber-Bosch Process
The Haber-Bosch Process involves the conversion of nitrogen gas (N2) and hydrogen gas (H2) into ammonia (NH3) through a catalytic reaction. The reaction takes place in a reactor at high temperatures (around 400-500 degrees Celsius) and pressures (approximately 200-300 atmospheres). The catalyst used in this process is iron combined with small amounts of other elements like potassium and aluminum.
The nitrogen gas and hydrogen gas are fed into the reactor, where they come into contact with the catalyst. The high temperature and pressure conditions provide the necessary energy for the reaction to occur. The nitrogen gas and hydrogen gas molecules break apart, and their atoms recombine to form ammonia molecules.
Importance of the Haber-Bosch Process in Agriculture
The Haber-Bosch Process is a game-changer in agriculture as it enables the production of synthetic ammonia. Ammonia is a key component of fertilizers, which are essential for promoting healthy plant growth. Nitrogen is an essential nutrient for plants, and it is often the limiting factor for crop productivity.
Before the discovery of the Haber-Bosch Process, farmers relied on natural sources of nitrogen, such as animal manure and leguminous plants. However, these sources were often insufficient to meet the demand for nitrogen in agriculture. The Haber-Bosch Process revolutionized agriculture by providing a reliable and consistent source of nitrogen through the production of ammonia-based fertilizers.
With the use of ammonia-based fertilizers, farmers are able to enhance crop yields and improve food security. These fertilizers provide the necessary nitrogen for plants to grow, develop healthy roots, and produce higher yields. The Haber-Bosch Process has played a significant role in increasing global food production and supporting a growing population.
Environmental Impact of the Haber-Bosch Process
While the Haber-Bosch Process has undoubtedly contributed to increased food production, it also has environmental implications. The process requires large amounts of energy, primarily derived from fossil fuels, to maintain the high temperatures and pressures needed for the reaction.
The combustion of fossil fuels releases carbon dioxide (CO2), a greenhouse gas that contributes to climate change. Additionally, the process can result in the formation of nitrogen oxides (NOX), which are harmful pollutants. Steps are being taken to improve the energy efficiency of the process and reduce its environmental impact.
Conclusion
The Haber-Bosch Process is an essential chemical process in modern agriculture. By converting nitrogen gas into ammonia, it allows for the production of ammonia-based fertilizers that contribute to increased crop yields and global food production. However, it is important to be mindful of the environmental impact of this process and work towards more sustainable alternatives.
FAQs
1. Who discovered the Haber-Bosch Process?
Fritz Haber and Carl Bosch are credited with the discovery and development of the Haber-Bosch Process.
2. What is the role of ammonia in agriculture?
Ammonia is a key component of fertilizers and provides the essential nitrogen nutrient for plants, promoting healthy growth and high yields.
3. What are the high temperature and pressure conditions required for the Haber-Bosch Process?
The Haber-Bosch Process operates at temperatures of around 400-500 degrees Celsius and pressures of approximately 200-300 atmospheres.
4. How does the Haber-Bosch Process contribute to food production?
The Haber-Bosch Process enables the production of ammonia-based fertilizers, which help enhance crop yields and improve food security.
5. Are there any environmental concerns associated with the Haber-Bosch Process?
The process requires large amounts of energy, often derived from fossil fuels, leading to carbon emissions and potential environmental pollution.